Vaccinations have prevented millions of infectious
illnesses, hospitalizations and deaths among U.S. children, yet the
long-term health outcomes of the vaccination schedule remain uncertain.
Studies have been recommended by the U.S. Institute of Medicine to
address this question. This study aimed 1) to compare vaccinated and
unvaccinated children on a broad range of health outcomes, and 2) to
determine whether an association found between vaccination and
neurodevelopmental disorders (NDD), if any, remained significant after
adjustment for other measured factors. A cross-sectional study of
mothers of children educated at home was carried out in collaboration
with homeschool organizations in four U.S. states: Florida, Louisiana,
Mississippi and Oregon. Mothers were asked to complete an anonymous
online questionnaire on their 6- to 12-year-old biological children with
respect to pregnancy-related factors, birth history, vaccinations,
physician-diagnosed illnesses, medications used, and health services.
NDD, a derived diagnostic measure, was defined as having one or more of
the following three closely-related diagnoses: a learning disability,
Attention Deficient Hyperactivity Disorder, and Autism Spectrum
Disorder. A convenience sample of 666 children was obtained, of which
261 (39%) were unvaccinated. The vaccinated were less likely than the
unvaccinated to have been diagnosed with chickenpox and pertussis, but
more likely to have been diagnosed with pneumonia, otitis media,
allergies and NDD. After adjustment, vaccination, male gender, and
preterm birth remained significantly associated with NDD. However, in a
final adjusted model with interaction, vaccination but not preterm birth
remained associated with NDD, while the interaction of preterm birth
and vaccination was associated with a 6.6-fold increased odds of NDD
(95% CI: 2.8, 15.5). In conclusion, vaccinated homeschool children were
found to have a higher rate of allergies and NDD than unvaccinated
homeschool children. While vaccination remained significantly associated
with NDD after controlling for other factors, preterm birth coupled
with vaccination was associated with an apparent synergistic increase in
the odds of NDD. Further research involving larger, independent samples
and stronger research designs is needed to verify and understand these
unexpected findings in order to optimize the impact of vaccines on
children’s health.
acute diseases, chronic diseases, epidemiology,
evaluation, health policy, immunization, neurodevelopmental disorders,
vaccination
Abbreviations:
ADHD: Attention Deficit Hyperactivity Disorder;
ASD: Autism Spectrum Disorder; AOM: Acute Otitis Media; CDC: Centers for
Disease Control and Prevention; CI: Confidence Interval; NDD:
Neurodevelopmental Disorders; NHERI: National Home Education Research
Institute; OR: Odds Ratio; PCV-7: Pneumococcal Conjugate Vaccine-7;
VAERS: Vaccine Adverse Events Reporting System.
Vaccines are among the greatest achievements of
biomedical science and one of the most effective public health
interventions of the 20th century [1]. Among U.S. children born between
1995 and 2013, vaccination is estimated to have prevented 322 million
illnesses, 21 million hospitalizations and 732,000 premature deaths,
with overall cost savings of $1.38 trillion [2]. About 95% of U.S.
children of kindergarten age receive all of the recommended vaccines as a
requirement for school and daycare attendance [3,4], aimed at
preventing the occurrence and spread of targeted infectious diseases
[5]. Advances in biotechnology are contributing to the development of
new vaccines for widespread use [6].
Under the currently recommended pediatric
vaccination schedule [7], U.S. children receive up to 48 doses of
vaccines for 14 diseases from birth to age six years, a figure that has
steadily increased since the 1950s, most notably since the Vaccines for
Children program was created in 1994. The Vaccines for Children program
began with vaccines targeting nine diseases: diphtheria, tetanus,
pertussis, polio, Haemophilus influenzae type b disease,
hepatitis B, measles, mumps, and rubella. Between 1995 and 2013, new
vaccines against five other diseases were added for children age 6 and
under: varicella, hepatitis A, pneumococcal disease, influenza, and
rotavirus vaccine.
Although short-term immunologic and safety testing
is performed on vaccines prior to their approval by the U.S. Food and
Drug Administration, the long-term effects of individual vaccines and of
the vaccination program itself remain unknown [8]. Vaccines are
acknowledged to carry risks of severe acute and chronic adverse effects,
such as neurological complications and even death [9], but such risks
are considered so rare that the vaccination program is believed to be
safe and effective for virtually all children [10].
There are very few randomized trials on any
existing vaccine recommended for children in terms of morbidity and
mortality, in part because of ethical concerns involving withholding
vaccines from children assigned to a control group. One exception, the
high-titer measles vaccine, was withdrawn after several randomized
trials in west Africa showed that it interacted with the
diphtheria-tetanus-pertussis vaccine, resulting in a significant 33%
increase in child mortality [11]. Evidence of safety from observational
studies includes a limited number of vaccines, e.g., the measles, mumps
and rubella vaccine, and hepatitis B vaccine, but none on the childhood
vaccination program itself. Knowledge is limited even for vaccines with a
long record of safety and protection against contagious diseases [12].
The safe levels and long-term effects of vaccine ingredients such as
adjuvants and preservatives are also unknown [13]. Other concerns
include the safety and cost-effectiveness of newer vaccines against
diseases that are potentially lethal for individuals but have a lesser
impact on population health, such as the group B meningococcus vaccine
[14].
Knowledge of adverse events following vaccinations
is largely based on voluntary reports to the Vaccine Adverse Events
Reporting System (VAERS) by physicians and parents. However, the rate of
reporting of serious vaccine injuries is estimated to be <1% [15].
These considerations led the former Institute of Medicine (now the
National Academy of Medicine) in 2005 to recommend the development of a
five-year plan for vaccine safety research by the Centers for Disease
Control and Prevention (CDC) [16,17]. In its 2011 and 2013 reviews of
the adverse effects of vaccines, the Institute of Medicine concluded
that few health problems are caused by or associated with vaccines, and
found no evidence that the vaccination schedule was unsafe [18,19].
Another systematic review, commissioned by the US Agency for Healthcare
Research and Quality to identify gaps in evidence on the safety of the
childhood vaccination program, concluded that severe adverse events
following vaccinations are extremely rare [20]. The Institute of
Medicine, however, noted that studies were needed: to compare the health
outcomes of vaccinated and unvaccinated children; to examine the
long-term cumulative effects of vaccines; the timing of vaccination in
relation to the age and condition of the child; the total load or number
of vaccines given at one time; the effect of other vaccine ingredients
in relation to health outcomes; and the mechanisms of vaccine-associated
injury [19].
A complicating factor in evaluating the vaccination
program is that vaccines against infectious diseases have complex
nonspecific effects on morbidity and mortality that extend beyond
prevention of the targeted disease. The existence of such effects poses a
challenge to the assumption that individual vaccines affect the immune
system independently of each other and have no physiological effect
other than protection against the targeted pathogen [21]. The
nonspecific effects of some vaccines appear to be beneficial, while in
others they appear to increase morbidity and mortality [22,23]. For
instance, both the measles and Bacillus Calmette–Guérin vaccine
reportedly reduce overall morbidity and mortality [24], whereas the
diphtheria-tetanus-pertussis [25] and hepatitis B vaccines [26] have the
opposite effect. The mechanisms responsible for these nonspecific
effects are unknown but may involve inter alia: interactions
between vaccines and their ingredients, e.g., whether the vaccines are
live or inactivated; the most recently administered vaccine;
micronutrient supplements such as vitamin A; the sequence in which
vaccines are given; and their possible combined and cumulative effects
[21].
A major current controversy is the question of
whether vaccination plays a role in neurodevelopmental disorders (NDDs),
which broadly include learning disabilities, Attention Deficit
Hyperactivity Disorder (ADHD) and Autism Spectrum Disorder (ASD). The
controversy has been fueled by the fact that the U.S. is experiencing
what has been described as a “silent pandemic” of mostly subclinical
developmental neurotoxicity, in which about 15% of children suffer from a
learning disability, sensory deficits, and developmental delays
[27,28]. In 1996 the estimated prevalence of ASD was 0.42%. By 2010 it
had risen to 1.47% (1 in 68), with 1 in 42 boys and 1 in 189 girls
affected [29]. More recently, based on a CDC survey of parents in
2011–2014, 2.24% of children (1 in 45) were estimated to have ASD. Rates
of other developmental disabilities, however, such as intellectual
disability, cerebral palsy, hearing loss, and vision impairments, have
declined or remained unchanged [30]. Prevalence rates of Attention
Deficit Hyperactivity Disorder (ADHD) have also risen markedly in recent
decades [31]. Earlier increases in the prevalence of learning
disability have been followed by declining rates in most states,
possibly due to changes in diagnostic criteria [32].
It is believed that much of the increase in NDD
diagnoses in recent decades has been due to growing awareness of autism
and more sensitive screening tools, and hence to greater numbers of
children with milder symptoms of autism. But these factors do not
account for all of the increase [33]. The geographically widespread
increase in ASD and ADHD suggests a role for an environmental factor to
which virtually all children are exposed. Agricultural chemicals are a
current focus of research [34-37].
A possible contributory role for vaccines in the
rise in NDD diagnoses remains unknown because data on the health
outcomes of vaccinated and unvaccinated children are lacking. The need
for such studies is suggested by the fact that the Vaccine Injury
Compensation Program has paid $3.2 billion in compensation for vaccine
injury since its creation in 1986 [38]. A study of claims compensated by
the Vaccine Injury Compensation Program for vaccine-induced
encephalopathy and seizure disorder found 83 claims that were
acknowledged as being due to brain damage. In all cases it was noted by
the Court of Federal Claims, or indicated in settlement agreements, that
the children had autism or ASD [39]. On the other hand, numerous
epidemiological studies have found no association between receipt of
selected vaccines (in particular the combined measles, mumps, and
rubella vaccine) and autism [10,40-45], and there is no accepted
mechanism by which vaccines could induce autism [46].
A major challenge in comparing vaccinated and
unvaccinated children has been to identify an accessible pool of
unvaccinated children, since the vast majority of children in the U.S.
are vaccinated. Children educated at home (“homeschool children”) are
suitable for such studies as a higher proportion are unvaccinated
compared to public school children [47]. Homeschool families have an
approximately equal median income to that of married-couple families
nationwide, somewhat more years of formal education, and a higher
average family size (just over three children) compared to the national
average of just over two children [48-50]. Homeschooling families are
slightly overrepresented in the south, about 23% are nonwhite, and the
age distribution of homeschool children in grades K-12 is similar to
that of children nationwide [51]. About 3% of the school-age population
was homeschooled in the 2011-2012 school year [52].
The aims of this study were 1) to compare
vaccinated and unvaccinated children on a broad range of health
outcomes, including acute and chronic conditions, medication and health
service utilization, and 2) to determine whether an association found
between vaccination and NDDs, if any, remained significant after
adjustment for other measured factors.
Study planning
To implement the study, a partnership was formed
with the National Home Education Research Institute (NHERI), an
organization that has been involved in educational research on
homeschooling for many years and has strong and extensive contacts with
the homeschool community throughout the country (www.nheri.org). The
study protocol was approved by the Institutional Review Board of Jackson
State University.
Study design
The study was designed as a cross-sectional survey
of homeschooling mothers on their vaccinated and unvaccinated biological
children ages 6 to 12. As contact information on homeschool families
was unavailable, there was no defined population or sampling frame from
which a randomized study could be carried out, and from which response
rates could be determined. However, the object of our pilot study was
not to obtain a representative sample of homeschool children but a
convenience sample of unvaccinated children of sufficient size to test
for significant differences in outcomes between the groups.
We proceeded by selecting 4 states (Florida,
Louisiana, Mississippi, and Oregon) for the survey (Stage 1). NHERI
compiled a list of statewide and local homeschool organizations,
totaling 84 in Florida, 18 in Louisiana, 12 in Mississippi and 17 in
Oregon. Initial contacts were made in June 2012. NHERI contacted the
leaders of each statewide organization by email to request their
support. A second email was then sent, explaining the study purpose and
background, which the leaders were asked to forward to their members
(Stage 2). A link was provided to an online questionnaire in which no
personally identifying information was requested. With funding limited
to 12 months, we sought to obtain as many responses as possible,
contacting families only indirectly through homeschool organizations.
Biological mothers of children ages 6-12 years were asked to serve as
respondents in order to standardize data collection and to include data
on pregnancy-related factors and birth history that might relate to the
children's current health. The age-range of 6 to 12 years was selected
because most recommended vaccinations would have been received by then.
Recruitment and informed consent
Homeschool leaders were asked to sign Memoranda of
Agreement on behalf of their organizations and to provide the number of
member families. Non-responders were sent a second notice but few
provided the requested information. However, follow-up calls to the
leaders suggested that all had contacted their members about the study.
Both the letter to families and the survey questions were stated in a
neutral way with respect to vaccines. Our letter to parents began:
“Dear Parent, This study concerns a major
current health question: namely, whether vaccination is linked in any
way to children's long-term health. Vaccination is one of the greatest
discoveries in medicine, yet little is known about its long-term impact.
The objective of this study is to evaluate the effects of vaccination
by comparing vaccinated and unvaccinated children in terms of a number
of major health outcomes …”
Respondents were asked to indicate their consent to
participate, to provide their home state and zip code of residence, and
to confirm that they had biological children 6 to 12 years of age. The
communications company Qualtrics (http://qualtrics.com) hosted the
survey website. The questionnaire included only closed-ended questions
requiring yes or no responses, with the aim of improving both response
and completion rates.
A number of homeschool mothers volunteered to
assist NHERI promote the study to their wide circles of homeschool
contacts. A number of nationwide organizations also agreed to promote
the study in the designated states. The online survey remained open for
three months in the summer of 2012. Financial incentives to complete the
survey were neither available nor offered.
Definitions and measures
Vaccination status was classified as unvaccinated
(i.e., no previous vaccinations), partially vaccinated (received some
but not all recommended vaccinations) and fully vaccinated (received all
recommended age-appropriate vaccines), as reported by mothers. These
categories were developed on the premise that any long-term effects of
vaccines would be more evident in fully-vaccinated than in
partially-vaccinated children, and rare or absent in the unvaccinated.
Mothers were asked to use their child’s vaccination records to indicate
the recommended vaccines and doses their child had received. Dates of
vaccinations were not requested in order not to overburden respondents
and to reduce the likelihood of inaccurate reporting; nor was
information requested on adverse events related to vaccines, as this was
not our purpose. We also did not ask about dates of diagnoses because
chronic illnesses are often gradual in onset and made long after the
appearance of symptoms. Since most vaccinations are given before age 6,
vaccination would be expected to precede the recognition and diagnosis
of most chronic conditions.
Mothers were asked to indicate on a list of more
than 40 acute and chronic illnesses all those for which her child or
children had received a diagnosis by a physician. Other questions
included the use of health services and procedures, dental check-ups,
“sick visits” to physicians, medications used, insertion of ventilation
ear tubes, number of days in the hospital, the extent of physical
activity (number of hours the child engaged in “vigorous” activities on a
typical weekday), number of siblings, family structure (mother and
father living in the home, divorced or separated), family income and/or
highest level of education of mother or father, and social interaction
with children outside the home (i.e., amount of time spent in
play or other contact with children outside the household). Questions
specifically for the mother included pregnancy-related conditions and
birth history, use of medications during pregnancy, and exposure to an
adverse environment (defined as living within 1-2 miles of a furniture
manufacturing factory, hazardous waste site, or lumber processing
factory). NDD, a derived diagnostic category, was
defined as having one or more of the following three closely related and
overlapping diagnoses: a learning disability, Attention Deficit
Hyperactivity Disorder (ADHD) and Autism Spectrum Disorder (ASD) [53].
Statistical methods
Unadjusted bivariate analyses using chi-square
tests were performed initially to test the null hypothesis of no
association between vaccination status and health outcomes, i.e.,
physician-diagnosed acute and chronic illnesses, medications, and the
use of health services. In most analyses, partially and fully vaccinated
children were grouped together as the “vaccinated” group, with
unvaccinated children as the control group. The second aim of the study
was to determine whether any association found between vaccination and
neurodevelopmental disorders remained significant after controlling for
other measured factors. Descriptive statistics on all variables were
computed to determine frequencies and percentages for categorical
variables and means (± SD) for continuous variables. The strength of
associations between vaccination status and health outcomes were tested
using odds ratios (OR) and 95% Confidence Intervals (CI). Odds ratios
describe the strength of the association between two categorical
variables measured simultaneously and are appropriate measures of that
relationship in a cross-sectional study [54]. Unadjusted and adjusted
logistic regression analyses were carried out using SAS (Version 9.3) to
determine the factors associated with NDDs.
Socio-Demographic characteristics of respondents
The information contained in 415 questionnaires
provided data on 666 homeschool children. Table 1 shows the
characteristics of the survey respondents. Mothers averaged about 40
years of age, were typically white, college graduates, with household
incomes between $50,000 to $100,000, Christian, and married. The reasons
for homeschooling for the majority of respondents (80-86%) were for a
moral environment, better family relationships, or for more contact with
their child or children.
Table 1. Characteristics of the respondentsa
|
Mean (SD) a |
Age (n=407) |
40.59 (6.7) |
|
Number (%)a |
Race |
|
White |
382 (92.5%) |
Non-White |
21 (7.6%) |
Total |
413 |
Education |
|
High School Graduate or Less |
35 (8.5%) |
Some College |
114 (27.5%) |
College Graduate |
187 (45.2%) |
Post-Graduates |
78 (18.5%) |
Total |
414 |
Total Gross Household Income |
|
< $49,999 |
123 (30.8%) |
$50,000-100,000 |
182 (45.5%) |
> $100,000 |
95 (23.8%) |
Total |
400 |
Religious Affiliation |
|
Christianity |
375 (91.2%) |
Non-Christianity |
36 (8.8%) |
Total |
411 |
Marital Status |
|
Married |
386 (93.7%) |
Not Married |
26 (6.3%) |
Total |
412 |
a Missing observations are excluded.
The children as a group were similarly mostly white
(88%), with a slight preponderance of females (52%), and averaged 9
years of age. With regard to vaccination status, 261 (39%) were
unvaccinated, 208 (31%) were partially vaccinated, and 197 (30%) had
received all of the recommended vaccinations. All statistical analyses
are based on these numbers.
Acute illness
Vaccinated children (N=405), combining the
partially and fully vaccinated, were significantly less likely than the
unvaccinated to have had chickenpox (7.9% vs. 25.3%, p <0.001; Odds
Ratio = 0.26, 95% Confidence Interval: 0.2, 0.4) and whooping cough
(pertussis) (2.5% vs. 8.4%, p <0.001; OR 0.3, 95% CI: 0.1, 0.6), and
less likely, but not significantly so, to have had rubella (0.3% vs.
1.9%, p = 0.04; OR 0.1, 95% CI: 0.01, 1.1). However, the vaccinated were
significantly more likely than the unvaccinated to have been diagnosed
with otitis media (19.8% vs. 5.8%, p <0.001; OR 3.8, 95% CI: 2.1,
6.6) and pneumonia (6.4% vs. 1.2%, p = 0.001; OR 5.9, 95% CI: 1.8,
19.7). No significant differences were seen with regard to hepatitis A
or B, high fever in the past 6 months, measles, mumps, meningitis (viral
or bacterial), influenza, or rotavirus (Table 2).
Table 2. Vaccination status and health outcomes – Acute Conditions
|
Vaccinated (n=405) |
Unvaccinated (n=261) |
Total (n=666) |
Chi-square |
P-value |
Odds Ratio
(95% CI) |
Chickenpox |
|
|
|
|
|
|
Yes |
32 (7.9%) |
66 (25.3%) |
98 (14.7%) |
38.229 |
< 0.001 |
0.26 (0.2 - 0.4) |
No |
373 (92.1%) |
195 (74.7%) |
568 (85.3%) |
Otitis media |
|
|
|
|
|
|
Yes |
80 (19.8%) |
16(5.8%) |
96 (14.4%) |
26.643 |
< 0.001 |
3.8 (2.1 - 6.6) |
No |
325 (80.2%) |
245 (94.2%) |
507 (85.6%) |
Pneumonia |
|
|
|
|
|
|
Yes |
26 (6.4%) |
3 (1.2%) |
29 (4.4%) |
10.585 |
< 0.001 |
5.9 (1.8 - 19.7) |
No |
379 (93.6%) |
258 (98.8%) |
637 (95.6%) |
Whooping cough |
|
|
|
|
|
|
Yes |
10 (2.5%) |
22 (8.4%) |
32 (4.8%) |
12.326 |
< 0.001 |
0.3 (0.1 - 0.6) |
No |
395 (97.5%) |
239 (91.6%) |
634 (95.2%) |
Rubella |
|
|
|
|
|
|
Yes |
1 (0.3%) |
5 (1.9%) |
6 (0.9%) |
4.951 |
0.037 |
0.1 (0.01 - 1.1) |
No |
404 (99.6%) |
256 (98.1%) |
660 (99.1%) |
Chronic illness
Vaccinated children were significantly more likely
than the unvaccinated to have been diagnosed with the following:
allergic rhinitis (10.4% vs. 0.4%, p <0.001; OR 30.1, 95% CI: 4.1,
219.3), other allergies (22.2% vs. 6.9%, p <0.001; OR 3.9, 95% CI:
2.3, 6.6), eczema/atopic dermatitis (9.5% vs. 3.6%, p = 0.035; OR 2.9,
95% CI: 1.4, 6.1), a learning disability (5.7% vs. 1.2%, p = 0.003; OR
5.2, 95% CI: 1.6, 17.4), ADHD (4.7% vs. 1.0%, p = 0.013; OR 4.2, 95% CI:
1.2, 14.5), ASD (4.7% vs. 1.0%, p = 0.013; OR 4.2, 95% CI: 1.2, 14.5),
any neurodevelopmental disorder (i.e., learning disability, ADHD or ASD)
(10.5% vs. 3.1%, p <0.001; OR 3.7, 95% CI: 1.7, 7.9) and any chronic
illness (44.0% vs. 25.0%, p <0.001; OR 2.4, 95% CI: 1.7, 3.3). No
significant differences were observed with regard to cancer, chronic
fatigue, conduct disorder, Crohn’s disease, depression, Types 1 or 2
diabetes, encephalopathy, epilepsy, hearing loss, high blood pressure,
inflammatory bowel disease, juvenile rheumatoid arthritis, obesity,
seizures, Tourette’s syndrome, or services received under the
Individuals with Disabilities Education Act (Table 3).
Table 3. Vaccination status and health outcomes – Chronic Conditions
Chronic Disease |
Vaccinated
(n=405) |
Unvaccinated
(n=261) |
Chi-square |
P-value |
Odds Ratio
(95% CI) |
Allergic rhinitis |
|
|
|
|
|
Yes |
42 (10.4%) |
1 (0.4%) |
26.21 |
< 0.001 |
30.1
(4.1 - 219.3) |
No |
363 (89.6%) |
260 (99.6%) |
Allergies |
|
|
|
|
|
Yes |
90 (22.2%) |
18 (6.9%) |
29.44 |
< 0.001 |
3.9
(2.3 - 6.6) |
No |
315 (77.9%) |
243 (93.1%) |
ADHD |
|
|
|
|
|
Yes |
19 (4.7%) |
3 (1.0%) |
6.23 |
0.013 |
4.2
(1.2 - 14.5) |
No |
386 (95.3%) |
258 (99.0%) |
ASD |
|
|
|
|
|
Yes |
19 (4.7%) |
3 (1.0%) |
6.23 |
0.013 |
4.2
(1.2 - 14.5) |
No |
386 (95.3%) |
258 (99.0%) |
Eczema (atopic dermatitis) |
|
|
|
|
|
Yes |
38 (9.5%) |
9 (3.6%) |
8.522 |
0.035 |
2.9
(1.4 - 6.1) |
No |
367 (90.5%) |
252 (96.4%) |
Learning Disability |
|
|
|
|
|
Yes |
23 (5.7%) |
3 (1.2%) |
8.6803 |
0.003 |
5.2
(1.6 - 17.4) |
No |
382 (94.3%) |
258 (98.9%) |
Neurodevelopment Disorder |
|
|
|
|
|
Yes |
42 (10.5%) |
8 (3.1%) |
12.198 |
< 0.001 |
3.7
(1.7 - 7.9) |
No |
313 (89.5%) |
253 (96.9%) |
Any Chronic Condition |
|
|
|
|
|
Yes |
178 (44.0%) |
65 (24.9%) |
24.8456 |
< 0.001 |
2.4
(1.7 - 3.3) |
No |
227 (56.0%) |
196 (75.1%) |
Partial versus full vaccination
Partially vaccinated children had an intermediate
position between the fully vaccinated and unvaccinated in regard to
several but not all health outcomes. For instance, as shown in Table 4,
the partially vaccinated had an intermediate (apparently detrimental)
position in terms of allergic rhinitis, ADHD, eczema, and learning
disability.
Table 4. Partial versus full vaccination and chronic health conditions
|
Unvaccinated (n=261) |
Partially Vaccinated (n=208) |
Fully Vaccinated (n=197) |
Total
(n=666) |
Chi-Square |
P-value |
Chronic Conditions |
|
|
|
|
|
|
Allergic rhinitis |
|
|
|
|
|
|
Yes |
1 (0.4%) |
17 (8.2%) |
25 (12.7%) |
43 (6.5%) |
29.6306 |
< 0.001 |
No |
260 (99.6%) |
191 (91.8%) |
172 (87.3%) |
623 (93.5%) |
Allergies |
|
|
|
|
|
|
Yes |
18 (6.9%) |
47 (22.6%) |
43 (21.8%) |
108 (16.2%) |
27.4819 |
< 0.001 |
No |
243 (93.1%) |
161 (77.4%) |
154 (78.2%) |
558 (83.8%) |
ADHD |
|
|
|
|
|
|
Yes |
3 (1.2%) |
8 (3.9%) |
11 (5.6%) |
22 (3.3%) |
7.1900 |
0.075 |
No |
258 (98.8%) |
200 (96.1%) |
186 (94.4%) |
644 (96.7%) |
ASD |
|
|
|
|
|
|
Yes |
3 (1.2%) |
11 (5.3%) |
8 (4.6%) |
22 (3.3%) |
6.7109 |
0.034 |
No |
258 (98.8%) |
197 (94.7%) |
189 (95.4%) |
644 (96.7%) |
Eczema (atopic dermatitis) |
|
|
|
|
|
|
Yes |
9 (3.5%) |
18 (8.7%) |
20 (10.2%) |
47 (7.1%) |
8.8683 |
0.012 |
No |
252 (96.5%) |
190 (91.3%) |
177 (89.8%) |
619 (92.9%) |
Learning Disability |
|
|
|
|
|
|
Yes |
3 (1.2%) |
11 (5.3%) |
12 (6.1%) |
26 (3.9%) |
8.8541 |
0.012 |
No |
258 (98.8%) |
197 (94.7%) |
185 (93.9%) |
640 (96.1%) |
NDD |
|
|
|
|
|
|
Yes |
8 (3.1%) |
21 (10.1%) |
21 (10.7%) |
50 (7.5%) |
12.2443 |
0.002 |
No |
253 (96.9%) |
187 (89.9%) |
176 (89.3%) |
616 (92.5%) |
Any Chronic Condition |
|
|
|
|
|
|
Yes |
65 (24.9%) |
94 (45.2%) |
84 (42.6%) |
243 (36.5%) |
25.1301 |
< 0.001 |
No |
196 (75.1%) |
114 (54.8%) |
113 (57.4%) |
423 (63.5%) |
Gender differences in chronic illness
Among the vaccinated (combining partially and fully
vaccinated children), boys were more likely than girls to be diagnosed
with a chronic condition – significantly so in the case of allergic
rhinitis (13.9% vs. 7.2%, p = 0.03; OR 2.1, 95% CI: 1.1, 4.1), ASD (7.7%
vs. 1.9%, p = 0.006; OR 4.3, 95% CI: 1.4, 13.2), and any
neurodevelopmental disorder (14.4% vs. 6.7%, p = 0.01; OR 2.3, 95% CI:
1.2, 4.6) (Table 5).
Table 5. Chronic conditions and gender among vaccinated children
|
Male
(n=194) |
Female
(n=209) |
Total
(n=403) |
Chi-square |
P-value |
Odds Ratio
(95% CI) |
Allergic rhinitis |
|
|
|
|
|
|
Yes |
27 (13.9%) |
15 (7.2%) |
42 (10.4%) |
4.8964 |
0.0269 |
2.1 (1.1 - 4.1) |
No |
167 (86.1%) |
194 (92.8%) |
361 (90.0%) |
Allergies |
|
|
|
|
|
|
Yes |
50 (25.8%) |
40 (19.1%) |
90 (22.3%) |
2.5531 |
0.1101 |
1.5 (0.91 - 2.4) |
No |
144 (74.2%) |
168 (80. 9%) |
313 (77.7%) |
ADHD |
|
|
|
|
|
|
Yes |
13 (6.7%) |
6 (2.9%) |
19 (4.7%) |
3.2856 |
0.0699 |
2.4 (0.90 - 6.5) |
No |
181 (93.3%) |
203 (97.1%) |
384 (95.3%) |
ASD |
|
|
|
|
|
|
Yes |
15 (7.7%) |
4 (1.9%) |
19 (4.7%) |
7.5810 |
0.0059 |
4.3 (1.4 - 13.2) |
No |
178 (92.3%) |
205 (98.1%) |
384 (95.3%) |
Eczema |
|
|
|
|
|
|
Yes |
19 (9.89%) |
19 (9.1%) |
38 (9.4%) |
0.0582 |
0.8094 |
1.1 (0.6 - 2.1) |
No |
175 (90.2%) |
190 (90.9%) |
365 (90.6%) |
Learning Disability |
|
|
|
|
|
|
Yes |
14 (7.2%) |
9 (4.3%) |
23 (5.7%) |
1.5835 |
0.2083 |
1.7 (0.7 - 4.1) |
No |
180 (92.8%) |
200 (95.7%) |
380 (94.3%) |
NDD |
|
|
|
|
|
|
Yes |
28 (14.4%) |
14 (6.7%) |
42 (10.4%) |
6.4469 |
0.0111 |
2.3 (1.2 - 4.6) |
No |
166 (85.6%) |
195 (93.3%) |
361 (89.6%) |
Any Chronic Condition |
|
|
|
|
|
|
Yes |
94 (48.5%) |
83 (39.7%) |
177 (43.9%) |
3.1208 |
0.0773 |
1.4 (1.0 - 2.1) |
No |
100 (51.5%) |
126 (60.3%) |
226 (56.1%) |
Use of medications and health services
The vaccinated (combining the partially and fully
vaccinated) were significantly more likely than the unvaccinated to use
medication for allergies (20.0% vs. 1.2%, p <0.001; OR 21.5, 95% CI:
6.7, 68.9), to have used antibiotics in the past 12 months (30.8% vs.
15.4%, p <0.001; OR 2.4, 95% CI: 1.6, 3.6), and to have used fever
medications at least once (90.7% vs. 67.8%, p <0.001; OR 4.6, 95% CI:
3.0, 7.1). The vaccinated were also more likely to have seen a doctor
for a routine checkup in the past 12 months (57.6% vs. 37.2%, p
<0.001; OR 2.3, 95% CI: 1.7, 3.2), visited a dentist during the past
year (89.4% vs. 80.5%, p <0.001; OR 2.0, 95% CI: 1.3, 3.2), visited a
doctor or clinic due to illness in the past year (36.0% vs. 16.0%, p
<0.001; OR 3.0, 95% CI: 2.0, 4.4), been fitted with ventilation ear
tubes (3.0% vs. 0.4%, p = 0.018; OR 8.0, 95% CI: 1.0, 66.1), and spent
one or more nights in a hospital (19.8% vs. 12.3%, p = 0.012; OR 1.8,
95% CI: 1.1, 2.7) (Table 6).
Table 6. Vaccination status, medication use and health services utilization
| |
Vaccinated
(n=405) |
Unvaccinated (n=261) |
Total
(n=666) |
Chi-square |
P-value |
Odds Ratio
(95% CI) |
Medication Use |
|
|
|
|
|
|
Medication for Allergy |
|
|
|
|
|
|
Yes |
81 (20.0%) |
3 (1.2%) |
84 (12.6%) |
51.170 |
< 0.001 |
21.5 (6.7 - 68.9) |
No |
324 (80.0%) |
258 (98.8%) |
582 (87.4%) |
Used antibiotics in the past 12 months |
|
|
|
|
|
|
Yes |
124 (30.8%) |
40 (15.4%) |
164 (24.7%) |
20.092 |
< 0.001 |
2.4 (1.6 - 3.6) |
No |
279 (69.2%) |
220 (84.6%) |
499 (75.3%) |
Used fever medication 1+ times |
|
|
|
|
|
|
Yes |
350 (90.7%) |
173 (67.8%) |
523 (81.6%) |
53.288 |
< 0.001 |
4.6 (3.0 - 7.1) |
No |
36 (9.3%) |
82 (32.2%) |
118 (18.4%) |
Using fitted ear drainage tubes |
|
|
|
|
|
|
Yes |
12 (3.0%) |
1 (0.4%) |
13 (2.0%) |
5.592 |
0.018 |
8.0 (1.0 - 66.1) |
No |
389 (97.0%) |
260 (99.6%) |
649 (98.0%) |
Used medication for ADHD |
|
|
|
|
|
|
Yes |
7 (1.7%) |
3 (1.2%) |
10 (1.5%) |
0.346 |
0.556 |
- |
No |
398 (98.3%) |
256 (98.8%) |
654 (98.5%) |
Used medication for Seizures |
|
|
|
|
|
|
Yes |
4 (1.0%) |
1 (0.4%) |
5 (0.8%) |
0.769 |
0.653 |
- |
No |
400 (99.0%) |
258 (99.6%) |
658 (99.2) |
Health Services
Utilization |
|
|
|
|
|
|
Emergency Department visit in the past 12 months |
|
|
|
|
|
|
Yes |
38 (9.5%) |
23 (9.0%) |
61 (9.3%) |
0.047 |
0.828 |
- |
No |
364 (90.5%) |
234 (91.0%) |
598 (90.7%) |
Sick visit to doctor in the past year |
|
|
|
|
|
|
Yes |
145 (36.0%) |
41 (16.0%) |
186 (28.2%) |
31.096 |
< 0.001 |
3.0 (2.0 - 4.4) |
No |
258 (64.0%) |
216 (84.0%) |
474 (71.8%) |
Ever spent one or more nights in the hospital |
|
|
|
|
|
|
Yes |
80 (19.8%) |
32 (12.3%) |
112 (16.8%) |
6.267 |
0.012 |
1.8 (1.1 - 2.7) |
No |
325 (80.2%) |
228 (87.7%) |
553 (83.2%) |
Seen doctor for checkup in past 12 months |
|
|
|
|
|
|
Yes |
233 (57.6%) |
97 (37.2%) |
330 (49.6%) |
26.336 |
< 0.001 |
2.3 (1.7 - 3.2) |
No |
172 (42.4%) |
164 (62.8%) |
336 (50.4%) |
Seen dentist in the past 12 months |
|
|
|
|
|
|
Yes |
362 (89.4%) |
210 (80.5%) |
572 (85.9%) |
10.424 |
< 0.001 |
2.0 (1.3 - 3.2) |
No |
43 (10.6%) |
51 (19.5%) |
94 (14.1%) |
Factors associated with neurodevelopmental disorders
The second aim of the study focused on a specific
health outcome and was designed to determine whether vaccination was
associated with neurodevelopmental disorders (NDD) and, if so, whether
the association remained significant after adjustment for other measured
factors. As noted, because of the relatively small numbers of children
with specific diagnoses, NDD was a derived variable combining children
with a diagnosis of one or more of ASD, ADHD and a learning disability.
The close association and overlap of these diagnoses in the study is
shown in the figure above (Figure 1). The figure shows that the single
largest group of diagnoses was learning disability (n=15) followed by
ASD (n=9), and ADHD (n=9), with smaller numbers comprising combinations
of the three diagnoses.
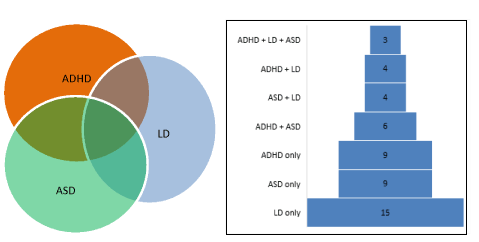
Figure 1. The overlap and distribution of physician-diagnosed neurodevelopmental disorders, based on mothers’ reports.
Unadjusted analysis
Table 7 shows that the factors associated with NDD
in unadjusted logistic regression analyses were: vaccination (OR 3.7,
95% CI: 1.7, 7.9); male gender (OR 2.1, 95% CI: 1.1, 3.8); adverse
environment, defined as living within 1-2 miles of a furniture
manufacturing factory, hazardous waste site, or lumber processing
factory (OR 2.9, 95% CI: 1.1, 7.4); maternal use of antibiotics during
pregnancy (OR 2.3, 95% CI: 1.1, 4.8); and preterm birth (OR 4.9, 95% CI:
2.4, 10.3). Two factors that almost reached statistical significance
were vaccination during pregnancy (OR 2.5, 95% CI: 1.0, 6.3) and three
or more fetal ultrasounds (OR 3.2, 95% CI: 0.92, 11.5). Factors that
were not associated with NDD in this study included mother’s education,
household income, and religious affiliation; use of acetaminophen,
alcohol, and antacids during pregnancy; gestational diabetes;
preeclampsia; Rhogham shot during pregnancy; and breastfeeding (data not
shown).
Table 7. Unadjusted analysis of potential risk factors for neurodevelopmental disorders
|
NDD |
Vaccination Status |
Yes
(N=50) |
No
(N=616) |
Total*
(N=666) |
Chi-Square |
P-value |
OR (95% CI)** |
Vaccinated |
42 |
363 |
405 |
12.198 |
<0.001 |
3.7 (1.7 - 7.9) |
Not Vaccinated |
8 |
253 |
261 |
Ref |
Race |
|
|
|
|
|
|
Non-White |
9 |
71 |
80 |
1.8208 |
0.177 |
1.7 (0.7 - 3.6) |
White |
41 |
544 |
585 |
Ref |
Child's Gender |
|
|
|
|
|
|
Male |
32 |
283 |
315 |
5.9471 |
0.015 |
2.1 (1.1 - 3.8) |
Female |
18 |
331 |
349 |
Ref |
Adverse Environment |
|
|
|
|
|
|
Yes |
6 |
27 |
33 |
5.8706 |
0.053 |
2.9 (1.1 - 7.4) |
No |
40 |
523 |
563 |
Ref |
Do not know |
4 |
66 |
70 |
0.8 (0.3 - 2.3) |
Medication during Pregnancy -
Antibiotics |
|
|
|
|
|
|
Yes |
10 |
61 |
71 |
4.950 |
0.026 |
2.3 (1.1 - 4.8) |
No |
40 |
555 |
595 |
Ref |
Medication during Pregnancy –
Vaccinated |
|
|
|
|
|
|
Yes |
6 |
32 |
38 |
3.965 |
0.057 |
2.5 (1.0 - 6.3) |
No |
44 |
583 |
627 |
Ref |
Preterm birth |
|
|
|
|
|
|
Yes |
12 |
37 |
49 |
22.910 |
< 0.001 |
4.9 (2.4 - 10.3) |
No |
38 |
578 |
616 |
Ref |
Ultrasound |
|
|
|
|
|
|
None |
3 |
71 |
74 |
5.898 |
0.052 |
Ref |
1-3 times |
30 |
419 |
449 |
- (0.5 - 5.7)
|
> 3 times |
17 |
124 |
141 |
|
|
3.2 (0.92 - 11.5) |
*Numbers may not add to column totals due to missing or incomplete data.
**Note that Odds Ratios are the cross-product ratios of the entries
in the 2-by-2 tables, and are an estimate of the relative incidence (or
risk) of the outcome associated with the exposure factor.
Adjusted analysis
After adjustment for all other significant factors,
those that remained significantly associated with NDD were: vaccination
(OR 3.1, 95% CI: 1.4, 6.8); male gender (OR 2.3, 95% CI: 1.2, 4.3); and
preterm birth (OR 5.0, 95% CI: 2.3, 11.1). The apparently strong
association between both vaccination and preterm birth and NDD suggested
the possibility of an interaction between these factors.
In a final adjusted model designed to test for this
possibility, controlling for the interaction of preterm birth and
vaccination, the following factors remained significantly associated
with NDD: vaccination (OR 2.5, 95% CI: 1.1, 5.6), nonwhite race (OR 2.4,
95% CI: 1.1, 5.4), and male gender (OR 2.3, 95% CI: 1.2, 4.4). Preterm
birth itself, however, was not significantly associated with NDD,
whereas the combination (interaction) of preterm birth and vaccination
was associated with 6.6-fold increased odds of NDD (95% CI: 2.8, 15.5)
(Table 8).
Table 8. Adjusted logistic regression analyses of risk factors and NDD*
|
Adjusted Model (Model 1) |
Adjusted Model with Interaction (Model 2) |
Vaccination Status |
|
|
Vaccinated |
3.1 (1.4 - 6.8) |
2.5 (1.1 - 5.6) |
Not Vaccinated |
Ref |
Ref |
Race |
|
|
Non-White |
2.3 (1.0 - 5.2) |
2.4 (1.1 - 5.4) |
White |
Ref |
Ref |
Child's Gender |
|
|
Male |
2.3 (1.2 - 4.3) |
2.3 (1.2 - 4.4) |
Female |
Ref |
Ref |
Preterm birth |
|
NS |
Yes |
5.0 (2.3 - 11.1) |
No |
Ref |
Preterm birth and Vaccination interaction |
|
|
No interaction |
Not in the model |
Ref |
Preterm and Vaccinated |
6.6 (2.8 - 15.5) |
*Number of observation read 666, number of observations used 629. NDD=47, Not NDD = 582
Following a recommendation of the Institute of
Medicine [19] for studies comparing the health outcomes of vaccinated
and unvaccinated children, this study focused on homeschool children
ages 6 to 12 years based on mothers’ anonymous reports of
pregnancy-related conditions, birth histories, physician-diagnosed
illnesses, medications and healthcare use. Respondents were mostly
white, married, and college-educated, upper income women who had been
contacted and invited to participate in the study by the leaders of
their homeschool organizations. Data from the survey were also used to
determine whether vaccination was associated specifically with NDDs, a
derived diagnostic category combining children with the diagnoses of
learning disability, ASD and/or ADHD.
With regard to acute and chronic conditions,
vaccinated children were significantly less likely than the unvaccinated
to have had chickenpox and pertussis but, contrary to expectation, were
significantly more likely to have been diagnosed with otitis media,
pneumonia, allergic rhinitis, eczema, and NDD. The vaccinated were also
more likely to have used antibiotics, allergy and fever medications; to
have been fitted with ventilation ear tubes; visited a doctor for a
health issue in the previous year, and been hospitalized. The reason for
hospitalization and the age of the child at the time were not
determined, but the latter finding appears consistent with a study of
38,801 reports to the VAERS of infants who were hospitalized or had died
after receiving vaccinations. The study reported a linear relationship
between the number of vaccine doses administered at one time and the
rate of hospitalization and death; moreover, the younger the infant at
the time of vaccination, the higher was the rate of hospitalization and
death [55]. The hospitalization rate increased from 11% for 2 vaccine
doses to 23.5% for 8 doses (r2 = 0.91), while the case
fatality rate increased significantly from 3.6% for those receiving from
1-4 doses to 5.4 % for those receiving from 5-8 doses.
In support of the possibility that the number of
vaccinations received could be implicated in risks of associated chronic
illness, a comparison of unvaccinated, partially and fully vaccinated
children in the present study showed that the partially vaccinated had
increased but intermediate odds of chronic disease, between those of
unvaccinated and fully vaccinated children, specifically for allergic
rhinitis, ADHD, eczema, a learning disability, and NDD as a whole.
The national rates of ADHD and LD are comparable to
those of the study. The U.S. rate of ADHD for ages 4-17 (twice the age
range of children than the present study), is 11% [31]. The study rate
of ADHD for ages 6 to 12 is 3.3%, and 4.7% when only vaccinated children
are included. The national LD rate is 5% [32], and the study data show a
rate of LD of 3.9% for all groups, and 5.6% when only vaccinated
children are included. However, the ASD prevalence of 2.24% from a CDC
parent survey is lower than the study rate of 3.3%. Vaccinated males
were significantly more likely than vaccinated females to have been
diagnosed with allergic rhinitis, and NDD. The percentage of vaccinated
males with an NDD in this study (14.4%) is consistent with national
findings based on parental responses to survey questions, indicating
that 15% of U.S. children ages 3 to 17 years in the years 2006-2008 had
an NDD [28]. Boys are also more likely than girls to be diagnosed with
an NDD, and ASD in particular [29].
Vaccination was strongly associated with both
otitis media and pneumonia, which are among the most common
complications of measles infection [56,57]. The odds of otitis media
were almost four-fold higher among the vaccinated (OR 3.8, 95% CI: 2.1,
6.6) and the odds of myringotomy with tube placement were eight-fold
higher than those of unvaccinated children (OR 8.0, 95% CI: 1.0, 66.1).
Acute otitis media (AOM) is a very frequent childhood infection,
accounting for up to 30 million physician visits each year in the U.S.,
and the most common reason for prescribing antibiotics for children
[58,59]. The incidence of AOM peaks at ages 3 to 18 months and 80% of
children have experienced at least one episode by 3 years of age. Rates
of AOM have increased in recent decades [60]. Worldwide, the incidence
of AOM is 10.9%, with 709 million cases each year, 51% occurring in
children under 5 years of age [61]. Pediatric AOM is a significant
concern in terms of healthcare utilization in the U.S., accounting for
$2.88 billion in annual health care costs [62].
Numerous reports of AOM have been filed with VAERS.
A search of VAERS for “Cases where age is under 1 and onset interval is
0 or 1 or 2 or 3 or 4 or 5 or 6 or 7 days and Symptom is otitis media”
[63] revealed that 438,573 cases were reported between 1990 and 2011,
often with fever and other signs and symptoms of inflammation and
central nervous system involvement. One study [64] assessed the
nasopharyngeal carriage of S. pneumoniae, H. influenzae, and M. catarrhalis
during AOM in fully immunized, partly immunized children with 0 or 1
dose of Pneumococcal Conjugate Vaccine-7 (PCV7), and “historical
control” children from the pre-PCV-7 era, and found an increased
frequency of M. catarrhalis colonization in the vaccinated
group compared to the partly immunized and control groups (76% vs. 62%
and 56%, respectively). A high rate of Moraxella catarrhalis colonization is associated with an increased risk of AOM [65].
Successful vaccination against pneumococcal
infections can lead to replacement of the latter in the nasopharyngeal
niche by nonvaccine pneumococcal serotypes and disease [66]. Vaccination
with PCV-7 has a marked effect on the complete microbiota composition
of the upper respiratory tract in children, going beyond shifts in the
distribution of pneumococcal serotypes and known potential pathogens and
resulting in increased anaerobes, gram-positive bacteria and
gram-negative bacterial species. PCV-7 administration also correlates
highly with the emergence and expansion of oropharyngeal types of
species. These observations have suggested that eradication of vaccine
serotype pneumococci can be followed by colonization of other bacterial
species in the vacant nasopharyngeal niche, leading to disequilibria of
bacterial composition (dysbiosis) and increased risks of otitis media.
Long-term monitoring has been recommended as essential for understanding
the full implications of vaccination-induced changes in microbiota
structure [67].
The second aim of the paper focused on a specific
health outcome and sought to determine whether vaccination remained
associated with neurodevelopmental disorders (NDD) after controlling for
other measured factors. After adjustment, the factors that remained
significantly associated with NDD were vaccination, nonwhite race, male
gender, and preterm birth. The apparently strong association between
both vaccination and preterm birth and NDD suggested the possibility of
an interaction between these factors. This was shown in a final adjusted
model with interaction (controlling for the interaction of preterm
birth with vaccination). In this model, vaccination, nonwhite race and
male gender remained associated with NDD, whereas preterm birth itself
was no longer associated with NDD. However, preterm birth combined with
vaccination was associated with a 6.6-fold increased odds of NDD.
In summary, vaccination, nonwhite race, and male
gender were significantly associated with NDD after controlling for
other factors. Preterm birth, although significantly associated with NDD
in unadjusted and adjusted analyses, was no longer associated with NDD
in the final model with interaction. However, preterm birth and
vaccination combined was strongly associated with NDD in the final
adjusted model with interaction, more than doubling the odds of NDD
compared to vaccination alone. Preterm birth has long been known as a
major factor for NDD [68,69], but since preterm infants are routinely
vaccinated, the separate effects of preterm birth and vaccination have
not been examined. The present study suggests that vaccination could be a
contributing factor in the pathogenesis of NDD but also that preterm
birth by itself may have a lesser or much reduced role in NDD (defined
here as ASD, ADHD and/or a learning disability) than currently believed.
The findings also suggest that vaccination coupled with preterm birth
could increase the odds of NDD beyond that of vaccination alone.
We did not set out to test a specific hypothesis
about the association between vaccination and health. The aim of the
study was to determine whether the health outcomes of vaccinated
children differed from those of unvaccinated homeschool children, given
that vaccines have nonspecific effects on morbidity and mortality in
addition to protecting against targeted pathogens [11]. Comparisons were
based on mothers’ reports of pregnancy-related factors, birth
histories, vaccinations, physician-diagnosed illnesses, medications, and
the use of health services. We tested the null hypothesis of no
difference in outcomes using chi-square tests, and then used Odds Ratios
and 96% Confidence Intervals to determine the strength and significance
of the association.
If the effects of vaccination on health were
limited to protection against the targeted pathogens, as is assumed to
be the case [21], no difference in outcomes would be expected between
the vaccinated and unvaccinated groups except for reduced rates of the
targeted infectious diseases. However, in this homogeneous sample of 666
children there were striking differences in diverse health outcomes
between the groups. The vaccinated were less likely to have had
chickenpox or whooping cough, as expected, but more likely to have been
diagnosed with pneumonia and ear infections as well as allergies and
NDDs.
What credence can be given to the findings? This
study was not intended to be based on a representative sample of
homeschool children but on a convenience sample of sufficient size to
test for significant differences in outcomes. Homeschoolers were
targeted for the study because their vaccination completion rates are
lower than those of children in the general population. In this respect
our pilot survey was successful, since data were available on 261
unvaccinated children.
To eliminate opportunities for subjectivity or
opinion in the data, only factual information was requested and the
questions involved memorable events such as physician-diagnosed diseases
in a child. With regard to minimizing potential bias in the information
provided by mothers, all communications with the latter emphasized
neutrality regarding vaccination and vaccine safety. To minimize recall
bias, respondents were asked to use their child’s vaccination records.
To enhance reliability, closed-ended questions were used and each set of
questions had to be completed before proceeding to the next. To enhance
validity, parents were asked to report only physician-diagnosed
illnesses.
Mothers’ reports could not be validated by clinical
records because the survey was designed to be anonymous. However,
self-reports about significant events provide a valid proxy for official
records when medical records and administrative data are unavailable
[70]. Had mothers been asked to provide copies of their children’s
medical records it would no longer have been an anonymous study and
would have resulted in few completed questionnaires. We were advised by
homeschool leaders that recruitment efforts would have been unsuccessful
had we insisted on obtaining the children’s medical records as a
requirement for participating in the study.
A further potential limitation is
under-ascertainment of disease in unvaccinated children. Could the
unvaccinated have artificially reduced rates of illness because they are
seen less often by physicians and would therefore have been less likely
to be diagnosed with a disease? The vaccinated were indeed more likely
to have seen a doctor for a routine checkup in the past 12 months (57.5%
vs. 37.1%, p < 0.001; OR 2.3, 95% CI: 1.7, 3.1). Such visits usually
involve vaccinations, which non-vaccinating families would be expected
to refuse. However, fewer visits to physicians would not necessarily
mean that unvaccinated children are less likely to be seen by a
physician if their condition warranted it. In fact, since unvaccinated
children were more likely to be diagnosed with chickenpox and whooping
cough, which would have involved a visit to the pediatrician,
differences in health outcomes are unlikely to be due to
under-ascertainment.
Strengths of the study include the unique design of
the study, involving homeschool mothers as respondents, and the
relatively large sample of unvaccinated children, which made it possible
to compare health outcomes across the spectrum of vaccination coverage.
Recruitment of biological mothers as respondents also allowed us to
test hypotheses about the role of pregnancy-related factors and birth
history as well as vaccination in NDD and other specific conditions. In
addition, this was a within-group study of a demographically homogeneous
population of mainly white, higher-income and college-educated
homeschooling families in which the children were all 6-12 years of age.
Information was provided anonymously by biological mothers, obviously
well-informed about their own children’s vaccination status and health,
which likely increased the validity of the reports.
Assessment of the long-term effects of the
vaccination schedule on morbidity and mortality has been limited [71].
In this pilot study of vaccinated and unvaccinated homeschool children,
reduced odds of chickenpox and whooping cough were found among the
vaccinated, as expected, but unexpectedly increased odds were found for
many other physician-diagnosed conditions. Although the cross-sectional
design of the study limits causal interpretation, the strength and
consistency of the findings, the apparent “dose-response” relationship
between vaccination status and several forms of chronic illness, and the
significant association between vaccination and NDDs all support the
possibility that some aspect of the current vaccination program could be
contributing to risks of childhood morbidity. Vaccination also remained
significantly associated with NDD after controlling for other factors,
whereas preterm birth, long considered a major risk factor for NDD, was
not associated with NDD after controlling for the interaction between
preterm birth and vaccination. In addition, preterm birth coupled with
vaccination was associated with an apparent synergistic increase in the
odds of NDD above that of vaccination alone. Nevertheless, the study
findings should be interpreted with caution. First, additional research
is needed to replicate the findings in studies with larger samples and
stronger research designs. Second, subject to replication, potentially
detrimental factors associated with the vaccination schedule should be
identified and addressed and underlying mechanisms better understood.
Such studies are essential in order to optimize the impact of
vaccination of children’s health.
Competing Interests
The authors declare that they have no financial
interests that had any bearing on any aspect of the conduct or
conclusions of the study and the submitted manuscript.
Author contributions
AM designed the study, contributed to data analysis
and interpretation, and drafted the paper. BR designed the study,
contributed to data collection, and edited the paper. AB contributed to
data analyses and edited the paper. BJ contributed to data analyses and
editing. All authors read and approved the final version of the paper.
Funding sources
This study was supported by grants from Generation
Rescue, Inc., and the Children’s Medical Safety Research Institute,
charitable organizations that support research on children’s health and
safety. The funders had no role or influence on the design and conduct
of the research or the preparation of reports.
The authors thank all those who contributed
critical comments, suggestions and financial support for the project. We
also thank the collaborating homeschool organizations and especially
the mothers who participated in the survey.
Disclaimer
This study was approved by the Institutional Review
Board of Jackson State University and completed prior to Dr. Mawson’s
tenure-track appointment at Jackson State University.
- Centers for Disease Control and Prevention (CDC) (1999) Ten great public health achievements--United States, 1900-1999. MMWR Morb Mortal Wkly Rep 48: 241-243. [Crossref]
- Whitney CG, Zhou F, Singleton J, Schuchat A;
Centers for Disease Control and Prevention (CDC) (2014) Benefits from
immunization during the vaccines for children program era - United
States, 1994-2013. MMWR Morb Mortal Wkly Rep 63: 352-355. [Crossref]
- Centers for Disease Control and Prevention (CDC)
(2007) Vaccination coverage among children in kindergarten--United
States, 2006-07 school year. MMWR Morb Mortal Wkly Rep 56: 819-821. [Crossref]
- Centers for Disease Control and Prevention (CDC)
(2013) Vaccination coverage among children in kindergarten - United
States, 2012-13 school year. MMWR Morb Mortal Wkly Rep 62: 607-612. [Crossref]
- http://www.cdc.gov/vaccines/vacgen/whatifstop.htm (Accessed 19 June 2016)
- http://www.hhs.gov/nvpo/vacc_plan/index.html (Accessed 19 June 2015).
- http://www.cdc.gov/vaccines/schedules/index.html (Accessed 19 June 2016).
- Ward BJ (2000) Vaccine adverse events in the new millennium: is there reason for concern? Bull World Health Organ 78: 205-215. [Crossref]
- Sienkiewicz D, Kulak W, Okurowska-Zawada B, Paszko-Pateg G (2012) Neurologic adverse events following vaccination. Prog Health Sci 2: 129-141.
- Pollard AJ (2007) Childhood immunisation: what is the future? Arch Dis Child 92: 426-433. [Crossref]
- [Crossref] Aaby P, Whittle H, Benn CS (2012) Vaccine programmes must consider their effect on general resistance. BMJ 344: e3769.
- [Crossref] Cunningham AS (2015) Vaccine mandates in the US are doing more harm than good. BMJ 351: h4576.
- Dórea JG. Exposure to mercury and aluminum in
early life: developmental vulnerability as a modifying factor in
neurologic and immunologic effects. Int J Environ Res Public Health (2015) 12(2):1295-313.
- [Crossref] Crowcroft NS1, Deeks SL2, Upshur RE2 (2015) Do we need a new approach to making vaccine recommendations? BMJ 350: h308.
- [Crossref]
Kessler DA1 (1993) Introducing MEDWatch. A new approach to reporting
medication and device adverse effects and product problems. JAMA 269: 2765-2768.
- http://www.nap.edu/catalog.php?record_id=11234 (Accessed 19 June 2016).
- http://www.cdc.gov/vaccinesafety/pdf/iso-finalscientific_agenda-nov- 10.pdf (Accessed 19 June 2016).
- Institute of Medicine (2012) Adverse Effects of
Vaccines: Evidence and Causality. The National Academies Press,
Washington, DC.
- Institute of Medicine (2013) The childhood
immunization schedule and safety: Stakeholder concerns, scientific
evidence, and future studies. The National Academies Press, Washington,
DC.
- Maglione MA, Das L, Raaen L, Smith A, Chari R,
et al. (2014) Safety of vaccines used for routine immunization of US
children: a systematic review. Pediatrics 134: 325-337. [Crossref]
- Siegrist CA (2008) Vaccine Immunology. Vaccines. (5th Edtn). Saunders Elsevier.
- Benn CS, Netea MG, Selin LK, Aaby P (2013) A small jab - a big effect: nonspecific immunomodulation by vaccines. Trends Immunol 34: 431-439. [Crossref]
- Jensen KJ, Benn CS, van Crevel R (2016)
Unravelling the nature of non-specific effects of vaccines - A challenge
for innate immunologists. Semin Immunol 28: 377-383. [Crossref]
- Sørup S, Benn CS, Poulsen A, Krause TG, Aaby P,
et al. (2014) Live vaccine against measles, mumps, and rubella and the
risk of hospital admissions for nontargeted infections. JAMA 311: 826-835. [Crossref]
- Aaby P, Benn C, Nielsen J, Lisse IM, Rodrigues
A, et al. (2012)Testing the hypothesis that diphtheria-tetanus-pertussis
vaccine has negative non-specific and sex-differential effects on child
survival in high-mortality countries. BMJ Open 2: e000707. [Crossref]
- Garly ML1, Jensen H, Martins CL, Balé C, Baldé
MA, et al. (2004) Hepatitis B vaccination associated with higher female
than male mortality in Guinea-Bissau: an observational study. Pediatr Infect Dis J 23: 10861092. [Crossref]
- Grandjean P, Landrigan PJ (2006) Developmental neurotoxicity of industrial chemicals. Lancet 368: 2167-2178. [Crossref]
- Boyle CA, Boulet S, Schieve LA, Cohen RA,
Blumberg SJ, , et al. (2011) Trends in the prevalence of developmental
disabilities in US Children, 1997-2008. Pediatrics 127: 10341042. [Crossref]
- Baio J (2014) Prevalence of Autism Spectrum
Disorder among children aged 8 years — Autism and Developmental
Disabilities Monitoring Network, 11 Sites, United States, 2010
Surveillance Summaries. MMWR 63: 1-21.
- Zablotsky B, Black LI, Maenner MJ, Schieve LA,
Blumberg SJ (2015) Estimated prevalence of autism and other
developmental disabilities following questionnaire changes in the 2014
National Health Interview Survey. Natl Health Stat Report 13: 1-20.
- Visser SN, Danielson ML, Bitsko RH, Holbrook JR,
Kogan MD, et al. (2014) Trends in the parent-report of health care
provider-diagnosed and medicated attention-deficit/hyperactivity
disorder: United States, 2003-2011. J Am Acad Child Adolesc Psychiatry 53: 34-46.e2. [Crossref]
- Cortiella C, Horowitz SH (2014) The State of
Learning Disabilities: Facts, Trends and Emerging Issues. National
Center for Learning Disabilities, New York:.
- Cornwall W (2015) Autism rates are up, but is it really on the rise? Science Magazine.
- Landrigan PJ (2010) What causes autism? Exploring the environmental contribution. Curr Opin Pediatr 22: 219-225. [Crossref]
- Nevison CD (2014) A comparison of temporal
trends in United States autism prevalence to trends in suspected
environmental factors. Environ Health 13: 73. [Crossref]
- Shaw CA, Seneff S, Kette SD, Tomljenovic L,
Oller JW Jr, et al. (2014) Aluminum-induced entropy in biological
systems: implications for neurological disease. J Toxicol 2014: 491316. [Crossref]
- Sealey LA, Hughes BW, Sriskanda AN1, Guest JR1,
Gibson AD1, et al. (2016) Environmental factors in the development of
autism spectrum disorders. Environ Int 88: 288-298. [Crossref]
- http://www.hrsa.gov/vaccinecompensation/data.html (Accessed 20 June 2016).
- Holland M, Conte L, Krakow R, Colin L (2011)
Unanswered questions from the Vaccine Injury Compensation Program: A
review of compensated cases of vaccine-induced brain injury. Pace Envtl L Rev 28: 480.
- Doja A, Roberts W (2006) Immunizations and autism: a review of the literature. Can J Neurol Sci 33: 341-346. [Crossref]
- Price CS, Thompson WW, Goodson B, Weintraub ES,
Croen LA, et al. (2010) Prenatal and infant exposure to thimerosal from
vaccines and immunoglobulins and risk of autism. Pediatrics 126: 656-664. [Crossref]
- DeStefano F, Price CS, Weintraub ES (2013)
Increasing exposure to antibody-stimulating proteins and polysaccharides
in vaccines is not associated with risk of autism. J Pediatr 163: 561-567. [Crossref]
- McNeil MM, Gee J, Weintraub ES, Belongia EA, Lee
GM, et al. (2014) The Vaccine Safety Datalink: successes and challenges
monitoring vaccine safety. Vaccine 32: 5390-5398. [Crossref]
- Taylor LE, Swerdfeger AL, Eslick GD (2014)
Vaccines are not associated with autism: an evidence-based meta-analysis
of case-control and cohort studies. Vaccine 32: 3623-3629. [Crossref]
- Jain A, Marshall J, Buikema A, Bancroft T, Kelly
JP, et al. (2015) Autism occurrence by MMR vaccine status among US
children with older siblings with and without autism. JAMA 313: 1534-1540. [Crossref]
- Gerber JS, Offit PA (2009) Vaccines and autism: a tale of shifting hypotheses. Clin Infect Dis 48: 456-461. [Crossref]
- Choi BK, Manning ML (2010) The immunization status of home-schooled children in America. J Pediatr Health Care 24: 42-47. [Crossref]
- Ray BD (2010) Academic achievement and demographic traits of homeschool students: a nationwide study. J Acad Leadership 8: 1.
- https://www.census.gov/library/publications/time-series/statistical_abstracts.html (Accessed 19 August 2016).
- http://files.eric.ed.gov/fulltext/ED505409.pdf (Accessed 22 August 2016).
- http://nces.ed.gov/pubs2006/2006042.pdf (Accessed 22 August 2016).
- http://eric.ed.gov/?id=ED544174 (Accessed 22 August 2016).
- Surén P, Bakken IJ, Aase H, Chin R, Gunnes N, et
al. (2012) Autism spectrum disorder, ADHD, epilepsy, and cerebral palsy
in Norwegian children. Pediatrics 130: e152-158. [Crossref]
- Zocchetti C, Consonni D, Bertazzi PA (1997)
Relationship between prevalence rate ratios and odds ratios in
cross-sectional studies. Int J Epidemiol 26: 220-223. [Crossref]
- Goldman GS, Miller NZ (2012) Relative trends in
hospitalizations and mortality among infants by the number of vaccine
doses and age, based on the Vaccine Adverse Event Reporting System
(VAERS), 1990-2010. Hum Exp Toxicol 31: 1012-1021. [Crossref]
- Orenstein WA, Perry RT, Halsey NA (2004) The clinical significance of measles: a review. J Infect Dis 189: S4-S16. [Crossref]
- CDC (2013) Prevention of measles, rubella,
congenital rubella syndrome, and mumps, 2013: Summary Recommendations of
the Advisory Committee on Immunization Practices (ACIP).
Recommendations and Reports. MMWR 62: 1-34.
- Dhooge IJ (2003) Risk factors for the development of otitis media. Curr Allergy Asthma Rep 3: 321-325. [Crossref]
- Siegel RM (2010) Acute otitis media guidelines, antibiotic use, and shared medical decision-making. Pediatrics 125: 384-386. [Crossref]
- Casselbrant ML, Mandel EM (2003) Epidemiology. Evidence-based otitis media. BC Decker, Hamilton, ON, Canada. Pp. 147–162.
- Monasta L1, Ronfani L, Marchetti F, Montico M,
Vecchi Brumatti L, et al. (2012) Burden of disease caused by otitis
media: systematic review and global estimates. PLoS One 7: e36226. [Crossref]
- Ahmed S1, Shapiro NL, Bhattacharyya N (2014) Incremental health care utilization and costs for acute otitis media in children. Laryngoscope 124: 301-305. [Crossref]
- http://www.medalerts.org/vaersdb/findfield.php?TABLE=ON&GROUP1=AGE&EVENTS=ON&SYMPTOMS[]=Otitis+media+%2810033078%29&NUMDAYS[]=0&NUMDAYS[]=1&NUMDAYS[]=2&NUMDAYS[]=3&NUMDAYS[]=4&NUMDAYS[]=5&NUMDAYS[]=6&NUMDAYS[]=7&WhicAge=range&LOWAGE=0.0&HIGHAGE=1.0)
(Accessed 25 August, 2016).
- Revai K, McCormick DP, Patel J, Grady JJ, Saeed
K, et al. (2006) Effect of pneumococcal conjugate vaccine on
nasopharyngeal bacterial colonization during acute otitis media. Pediatrics 117: 1823–1829. [Crossref]
- Faden H, Harabuchi Y, Hong JJ (1994)
Epidemiology of Moraxella catarrhalis in children during the first 2
years of life: relationship to otitis media. J Infect Dis 169: 1312-1317. [Crossref]
- Weinberger DM, Malley R, Lipsitch M (2011) Serotype replacement in disease after pneumococcal vaccination. Lancet 378: 1962-1973. [Crossref]
- Biesbroek G, Wang X, Keijser BJ, Eijkemans RM,
Trzcinski K, et al. (2014) Seven-valent pneumococcal conjugate vaccine
and nasopharyngeal microbiota in healthy children. Emerg Infect Dis 20: 201-210.
- Goldin RL, Matson JL (2016) Premature birth as a risk factor for autism spectrum disorder. Dev Neurorehabil 19: 203-206. [Crossref]
- Padilla N, Eklöf E, Mårtensson GE, Bölte S,
Lagercrantz H, et al. (2015) Poor brain growth in extremely
preterm neonates long before the onset of autism spectrum disorder
symptoms. Cereb Cortex 27: 1245-1252. [Crossref]
- Short ME, Goetzel RZ, Pei X, Tabrizi MJ,
Ozminkowski RJ, et al. (2009) How accurate are self-reports? Analysis of
self-reported health care utilization and absence when compared with
administrative data. J Occup Environ Med 51: 786-796. [Crossref]
- Fisker AB, Hornshøj L, Rodrigues A, Balde I,
Fernandes M, et al. (2014) Effects of the introduction of new vaccines
in Guinea-Bissau on vaccine coverage, vaccine timeliness, and child
survival: an observational study. Lancet Glob Health 2: e478-e487.

